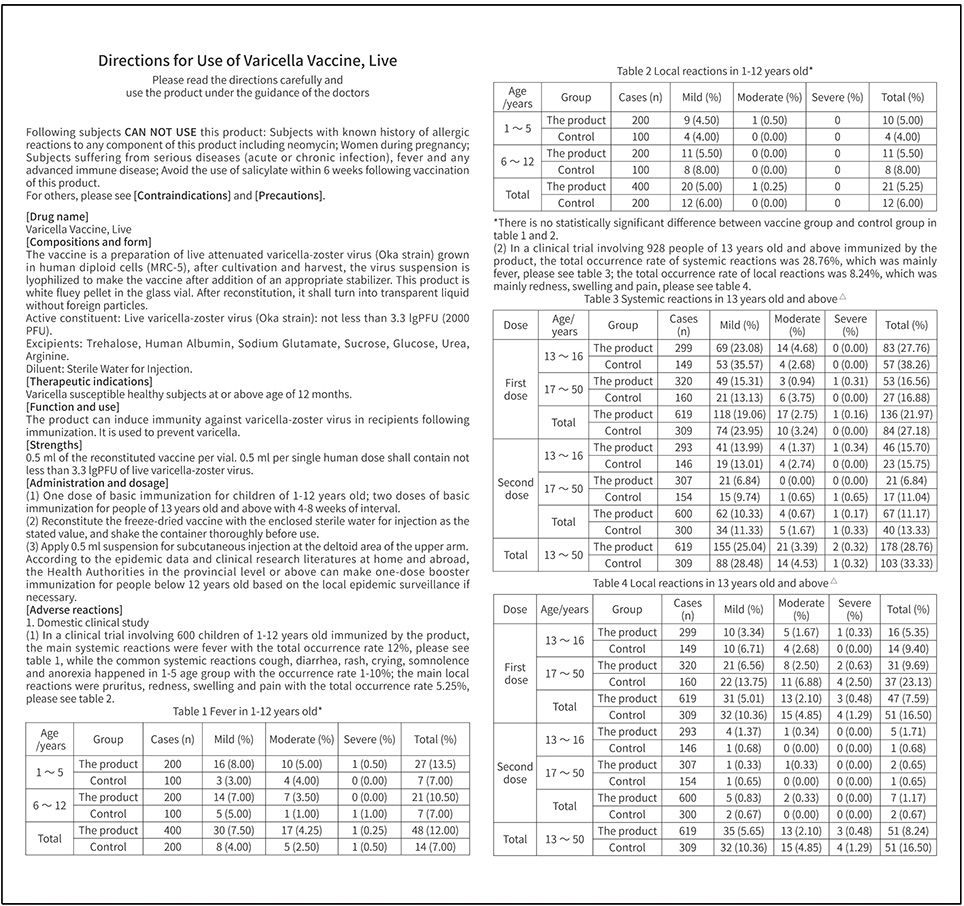
Product Name: Varicella Vaccine, Live
CFDA Approval No.: S20083005
BCHT’s Varicella Vaccine, Live adopting human diploid cells (MRC-5) as production cells and Oka strain as production strain, which are recommended by WHO. Our Varicella Vaccine, Live adopts BH-2 stabilizer with own IP rights, which greatly enhances the stability of the product and ensures the validity period for 36 months, the longest validity period in the world. This product owns the following characteristics:
1.Good safety of gelatin-free
Early foreign studies proved that most adverse reactions were related to gelatin. BCHT’s Varicella Vaccine, Live is the first gelatin-free varicella vaccine in China. Compared with the varicella vaccine containing gelatin, our Varicella Vaccine, Live can significantly reduce the incidence of allergic reaction.
2.Better protection with high titer and immune efficacy
It is documented that compared with the varicella vaccine of low titer, BCHT’s Varicella Vaccine, Live can reduce breakthrough cases by nearly 75%. Of all released batches of our Varicella Vaccine, Live, the virus titer are not less than 4.0 lgPFU/dose, and the virus titer within the validity period is not less than 3.3 lgPFU/dose.
3.Long validity period by good stability
The first approved varicella vaccine with validity period of 36 months in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6,International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.